Chapter 46: Coppice and Coppice-with-standards – Download PDF
Authors: Jan den Ouden, Patrick Jansen, Linda Meiresonne
Author affiliations are given at the end of the chapter
Intended learning level: Basic / Applied (Professional)
This material is published under Creative Commons license CC BY-NC-SA 4.0.
|
Purpose of the chapter: |
|---|
|
This chapter describes the coppice and coppice-with-standards systems that have been, and are, commonly applied in Europe. Some common management practices are explained. Most coppiced woodlands have been converted to high forest, but there is some renewed interest in these systems from nature conservation. |
NOTE: the figures in this chapter will be restyled in due time
Table of contents
46.2.5 Conversion and restoration of former coppice 9
46.2.6 Short rotation coppice 9
46.3 Coppice-with-standards 10
46.4 Natural values in coppice and coppice-with-standards 13
46. Coppice and Coppice-with-standards
Coppice is a form of forest management where stands are rejuvenated through vegetative regeneration of the stumps that remain after felling the trees (Figure 46-1). It is probably the oldest form of forest management, as many broadleaved trees will spontaneously produce new shoots that can be cut over and over again. The new shoots emerge from the stumps or root suckers from the root system and can be recut after some time. Coppice doesn’t require large capital investments and yields products on a regular basis, usually in small dimensions. The coppice system can be combined with large trees: the coppice-with-standards system (see 46.3).
Figure 46-1: Terminology related to coppicing. See also Rackham (2003) and Unrau et al. (2018) for a more detailed explanation and illustration of terms.
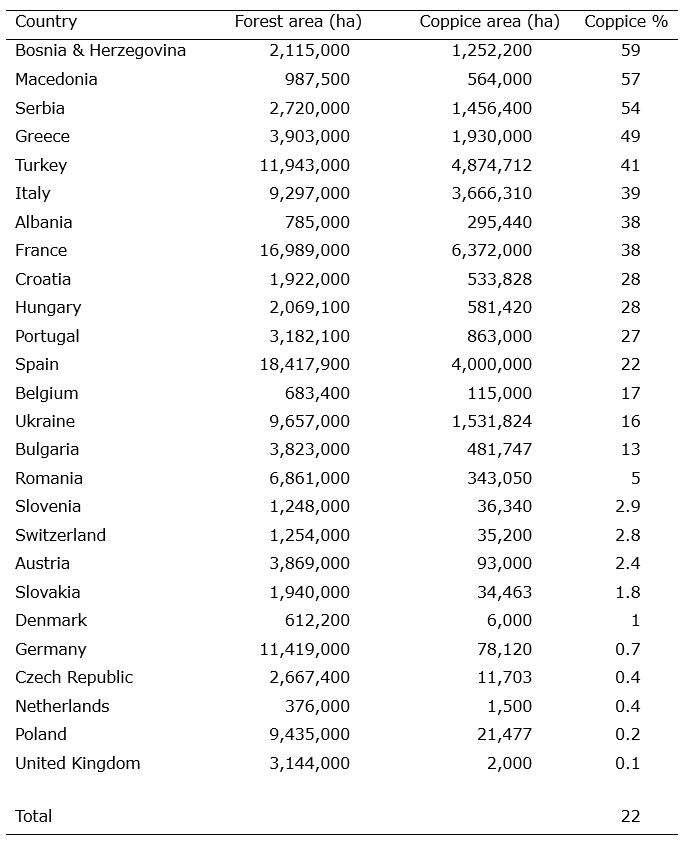
From the Middle Ages to early in the 20th century, coppicing was a wide-spread management system in Europe where forests managed as high forest (section 39) were relatively rare. More recently, the area of European coppiced forest has declined, yet, about 22% (>20 million ha) of the forest area (excluding Nordic and Baltic countries) is still under coppice, with the highest percentages found in south-east Europe and the Mediterranean where it still forms an important part of subsistence farming (Table 46-1).
Coppicing results in a multitude of relatively small-sized products making it easy to manage by manual labour, a great advantage in areas where few mechanized resources are available. Moreover, vegetative propagation is a much easier and more secure method of stand rejuvenation than sowing or planting (Rackham, 2006). At the beginning of the 21st century, the spotlight fell once again on coppicing as a cultural-historical management system with its associated diverse flora and fauna (Unrau et al., 2018). A modern version of coppicing is the ‘short rotation coppice’ system, primarily fit for the production of biofuels (section 46.2.6).
Table 46-1: Total forest area, coppice area, and percentage of forest under coppice (including coppice-with-standards) in European countries (excl. Nordic and Baltic states). Data compiled by Unrau et al. (2018).
46.1 Coppicing
After cutting the stem, shoots can regrow in different ways. In most cases they develop from dormant buds (section 12). However, in a number of species such as Fagus, Betula, Carpinus, Robinia, Ulmus and Aesculus, shoots can also arise from adventitious buds that develop in the wound tissue formed near the cut surface of the stump. This often produces weaker shoots than those developing from dormant buds. A limited number of species, including Alnus, Populus, Ulmus and Robinia, also readily regenerate through root suckers derived from adventitious buds on the roots. Initially, the new shoots use the existing root system, but they quickly establish their own new root system at the base of the new shoots.
In principle, any tree species able to resprout from the stump can be coppiced. This generally applies to broadleaved species; most coniferous trees do not have this capacity and are therefore unsuitable for coppicing. Literature on the suitability of European broadleaved species for coppice systems show regional differences; for example, Rackham (2006) regards beech as well suited for coppicing in the UK, while Boer (1857) regards beech less suitable in the Netherlands. An overview of the species used for coppicing in Europe is provided by Unrau et al. (2018). Commonly used species for coppicing throughout Europe include Quercus, Salix and Populus species, Castanea sativa, Fraxinus excelsior, Carpinus betulus.
46.1.1 Terminology
When a tree is cut for the first time, this leaves a stump on which new shoots or sprouts can develop (Figure 46-1). When these shoots are recut, new groups of shoots arise on several stumps connected to a joint stool.
There are different forms of coppicing, depending on the height of the stool (Figure 46-1). Typically, a coppice is cut as close to the ground as possible to facilitate the formation of a strong root system connected to the new shoots. To prevent cattle or game from eating the new shoots, or to prevent flooding, the stool can also be kept higher above the ground: this is called pollarding. The stool (pollard) is located at the top of a low stem which can rot early and, as a result, are often of little value. The most commonly pollarded tree is Salix, but Quercus, Fraxinus, Alnus and Populus can also be treated in this way. Another form of coppicing is shredding, where branches are cut along the trunk of a tree to harvest leaves and twigs for animal feedstock.
46.1.2 Products
Due to the short rotation periods (2-40 years), coppicing produces shoots and stems of relatively small proportions. Firewood is the main product, but coppicing also provides a variety of materials for many other uses, depending on the tree species and size, such as birch twigs to make brooms, stems to make tool handles, poles, fence posts, and willow branches and twigs for wickerwork and to braid fascine mattresses for waterworks (see Rackham 2003, Tack et al. 1993 and Unrau et al. 2018) for extensive overviews).
Until the twentieth century, large areas of oak coppice were cultivated to harvest tanbark for leather manufacturing. In some regions, the bark was beaten off the wood in spring, when the strong sap flow facilitated its detachment from the wood. Elsewhere, the bark was cut from the wood. The price and consequently use of oak bark fell sharply around the turn of the 19th century, when much improved vegetable tanning agents and, later, synthetic tanning agents became available.
All wood with no special purpose or unsuitable for other applications, may be used as firewood. A number of species are cultivated in coppice systems especially for this purpose, mainly Quercus, Alnus, Fagus and Carpinus. If coppiced in larger sizes, the wood can be turned into charcoal (Box 46.1 Firewood and Charcoal). In modern short rotation coppice systems, biomass is grown commercially for energy generation and as a raw material for the chip and paper industry (section 46.2.6).
|
Box 46.1 Firewood and Charcoal |
|---|
|
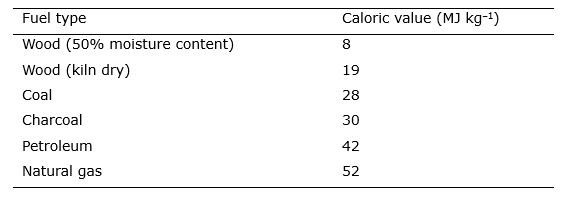
About half of the harvested wood world-wide is used as fuel. Before the emergence of (sub)fossil fuels such as peat, coal, oil and gas, wood was the most important fuel in Europe, and is still important in heavily forested and rural regions. As a fuel type, wood has a medium calorific value (heating value, see Table Box 46.1.1) but its energy efficiency strongly depends on the moisture content. The average calorific value of wood in a dry state is around 19 MJ kg–1 dry matter for European deciduous wood and 19.2 MJ kg–1 for European coniferous wood. Depending on the moisture content (mc%), expressed on a dry matter basis, the calorific value (CV) can be calculated using the formula CV(v%) = [ CV(v%=0) – 0.2164 * mc% ] where the constant 0.2164 reflects the energy required to evaporate water from the wood. Wood can be charred into charcoal. Charcoal weighs much less than wood and has twice the energy content, with an average of 27 MJ kg–1 for charcoal compared to 13 MJ kg–1 for wood with 25-30% moisture content. Charcoal therefore reduces transport costs and is suitable for activities that require high temperatures such as iron forging and glass making. Traditionally, charcoal was made in a charcoal kiln (see Photo Box 46.1.1). A pile of wood was stacked in a special way and covered with sods and a layer of moist, preferably loamy soil (about 20 cm thick) to prevent the entry of oxygen. The wood was ignited through a central shaft in the middle of the kiln and had to burn for days to char all the wood. When hardwood is charred in a kiln, 45-50% of the volume remains, but only 18-20% of the weight. A stacked meter (stère) of oak yields approximately 0.4 m3 charcoal (Anonymus, 1907). According to Rossier and Micuta (1999), 1 kg of charcoal requires 8-12 kg of wood. Modern charcoal production takes place in exterior-heated boilers. Table Box 46.1.1: Energy content of different types of fuels.
Photo Box 46.1.1. A charcoal kiln in full operation in the German Black Forest around 1910. By making holes in the layer of earth over the kiln, the oxygen supply and the temperature in the burning kiln can be accurately controlled. Photo: Karl Blumenthal. |
46.2 Coppice management
Coppice management varies by species, region, and desired product. Here, we only discuss the main points of contemporary and historical coppice systems. For a more detailed treatment we refer to, among others, Unrau et al. (2018), Boer (1857), Buis (1985), Mitchell et al. (1992), Tack et al. (1993), Danfors et al. (1998), Jansen and Kuiper (2001) and Rackham (2003, 2006). Harmer and Howe (2003) and Unrau et al. (2018) provide a practical overview of current coppice management.
There is evidence of coppicing in the Neolithic era, and it was widespread during Roman times (Rackham, 2003; Peterken, 1993). It is thought to have arisen gradually following felling of wild deciduous forest, where stumps were found to sprout again and again. From the Middle Ages onwards, many new coppice cultures were established in periods of wood shortage, population growth, and early industrialization.
46.2.1 Planting
Coppice forest is predominantly planted, but can also be ‘cultivated’ from existing vegetation, for example, naturally established oak on heathlands. To establish a new coppice, relatively large-sized planting stock obtained from nurseries or locally harvested young trees can be used. In willow or poplar coppices, planting cuttings suffices. Root suckers can also be used to start a new coppice cycle. Whatever the source, planting distance depends on soil fertility, the species, and the desired product. The better the soil and the larger the desired assortment, the greater the distance between plants. In general, new coppice is planted in spacing intervals comparable to those of regular forest plantations, leading to initial densities of 1000-5000 plants per ha. The resulting stool densities are lower due to mortality or deliberate thinning. When plants or stools die, they can be easily replaced after the next cutting cycle to maintain a homogeneous stand.
In young coppice cultures, production increases after the initial cutting cycles. Data from a historic coppice culture on the Entel in the Dutch Achterhoek show that production on a unit of coppice increased in the first five rotations (Schaars, 1974) where with a nine-year cutting cycle, the stool productivity did not decrease in the first 50 years. As stool productivity tends to decline after a number of cutting cycles, in current coppice systems stools are replaced after 3-5 cutting cycles (Unrau et al., 2018), depending on soil fertility and length of the cycle.
46.2.2 Rotation
The period between two harvests (rotation) depends on the size and dimensions of the product to be harvested (market demand) and site conditions. Typically, rotation periods in contemporary coppice vary between 15-40 years (Unrau et al., 2018). The better the soil and the more favourable the climate, the shorter the rotation period. The larger the desired wood, the longer the rotation period. For England, Rackham (2003) shows that from 1250-1850, the average rotation period increased from about 6 to 15 years. In the Netherlands, the rotation period of oak coppice seems to have increased between the 17th and 19th centuries (den Ouden et al., 2007), but no such extension of the rotation period was noted in Flanders (Tack et al., 1993). These changes in historic rotation periods reflect the needs of the economy and the demand for specific products. Soil depletion may also play a role in the extension of rotation periods in old coppice cultures on poorer soils (see Rackham, 2003). In addition, the arrival of oak mildew in the early 20th century slowed the growth of oak coppice to such an extent that rotation periods were extended.
46.2.3 Harvesting
When coppice is harvested, a plot (or coupe) is felled at once. When the number of coupes is equal to, or a multiple of, the number of years within the planned rotation period, it is possible to harvest continuously every year (Figure 46-2). In fact, this system formed the basis for forestry planning in plantation forests in the 19th and 20th centuries (see Hundeshagen, 1826). Harvesting preferably takes place in the winter, however oak coppicing for tanbark was an exception, being harvested in May or early June facilitating the removal of the bark.
Figure 46-2: Schematic overview of a coppice culture over time. The coppice is divided into four coupes, each with a 12 year rotation period. Shown on top are the situations at T=0 yrs and T=6 yrs. On T=0, the coupe on the lower left corner has just been cut. After two rounds of cutting (T=6) the age structure is identical to T=0, but the different coupes have a different age and structure. Over time, the shoots grow larger, but due to mortality, fewer shoots remain on a stool. After canopy closure, the amount of light reaching the forest floor is strongly reduced (lower graph).
The wood should be cut with sharp and clean tools and damage to the bark must be avoided. The surface of the cut stump should slope to ensure good drainage and prevent rot. The wood should be cut as close to the ground as possible to increase opportunities for the new shoots to develop an independent root system. The height at which the wood is cut has little influence on resprouting, as long as the wood is not cut below the point of attachment of the shoot to the old stump (Harmer, 2001).
46.2.4 Yield of coppice
It is difficult to exactly determine annual yields per unit area in historical coppice cultures, as this depended on the rotation period, soil fertility, planting conditions, and the age of the stools. The available data mainly relate to firewood and tanbark. In historical sources, yields from coppice are usually expressed in money or in units of faggots (bundles of twigs), firewood, etc. These are difficult to convert into contemporary units (m3 or kg), so we have little historic information about coppice productivity; some sources indicate 2-7 m3 ha–1 yr–1 (Harmer & Howe, 2003; Crockford & Savill, 1991). This concerns stem wood, but the actual amount of biomass removed is greater because branches and twigs can also be harvested. The annual growth rate of oak coppice is comparable to the growth of trees in high forest of the same age, but due to the higher density, the peak of volume growth is reached earlier (Crockford & Savill, 1991).
For France, Gannevat (1950) constructed a yield table for coppiced oak divided into different yield classes. On productive sites with a longer rotation period, an average production of up to 12 stère ha–1 yr–1 can be achieved, while in poor conditions, with coppice in shorter rotation cycles, the average production does not exceed 2 stère ha–1 yr –1 (Figure 46-3). This corresponds to an actual wood volume of approximately 1.5 – 9 m3 ha–1 yr–1. One stère represents a volume of a 1 m3 stack of wood although the actual wood volume in such a stack is smaller, about 0.7 m3, depending on the straightness of the logs.
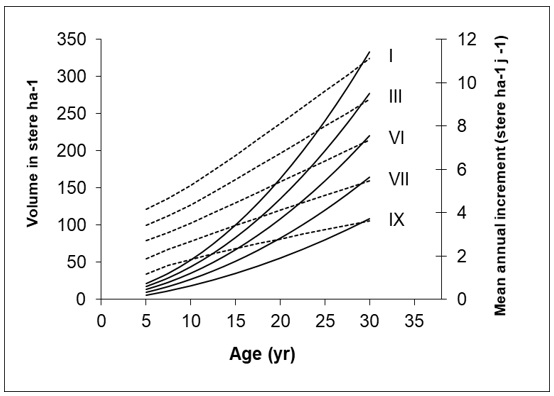
Figure 46-3: Volume (solid lines) and mean annual increment (dashed lines) in oak coppice of different yield classes (roman numbers) in France. Volumes expressed in stères (cubic metres of stacked wood). After Gannevat (1950).
46.2.5 Conversion and restoration of former coppice
Coppicing has declined sharply over the past century as a result of the reduced demand for firewood and bark and the increased costs of this labour-intensive form of management. In Great Britain, between 1905 and 1965, the area of coppice fell from 233,000 ha to 30,000 ha (Buckley, 1992). In Europe in the 20th century, the area of coppiced forest decreased significantly with most coppice cultures being converted to high forest or simply set aside. Traditional coppicing persisted in rural areas as part of subsistence farming.
Coppiced areas were usually converted by planting other species like Scots pine, Douglas fir, or larch directly among the coppice stools; in these forests remains of the old stools can often still be found. Conversion to high forest may also be the result of letting the shoots grow out to trees (conversion by aging) or by selecting one or two of the most powerful shoots some years after the coppice was last cut, and then cutting the remaining shoots (conversion by singling; Figure 46-1). After singling, the resulting trees are often not productive and generally have poor stem quality.
Coppice cultures are once again in the picture because of their associated natural and cultural-historical values. To restore a coppice system, cutting trees from the old stools often does not suffice as these old and thick stems only sprout to a limited extent, or not at all. The chance of sprouting is related to age; the number of dormant buds gradually decreases as the tree ages. Even if all newly cut stools resprout successfully, the remaining forest still has a low density because the original stem density decreased sharply in recent decades as a result of (self-) thinning. The forest canopy will then remain open for a long time, increasing the chance that the stand becomes overgrown by bramble or bracken. Therefore, to restore a coppice, it is usually necessary to replant trees, whether or not between the old stools. Controlling competing ground vegetation is also necessary to ensure that the young plants become sufficiently established and to encourage resprouting of the remaining stools. In the event of high browsing pressure, the young shoots and plants must also be protected against ungulates, for example by fencing.
46.2.6 Short rotation coppice
A recent development is the short rotation coppice. Here, dense plantings are used of tree species with rapid youth growth, especially Populus, Salix, Eucalyptus and Robinia, which are harvested every 2-5 years. The plant density depends on the species, rotation period and soil, with up to 10,000 – 20,000 plants per ha (Unrau et al. 2018). The planting design allows for access with machines for harvesting, weed control and fertilization. The high plant density ensures rapid crown closure to maximize productivity. Mixing different clones of vegetatively propagated species like willow or poplar spreads the risk of diseases and infestations.
When established on former agricultural fields, the first rotation(s) may be grown without fertilization, but after subsequent harvests, fertilizer can be applied to maintain productivity. A form of fertilization is the spread of combustion ash from the power plant where the wood is burned. Effective weed control during the first months after planting and after harvest is important to prevent smothering or crushing of the young plants or shoots, and where necessary, plantings must be protected against herbivores.
The growth culmination point in short-rotation coppice is reached after a few years, depending on species and planting density. This period is commonly held as the rotation period. After several rotations (up to 7-8), the stools are cleared and the site replanted. Annual production of short rotation coppicing can amount to a maximum of 8-40 tons of dry matter per hectare, depending on species, rotation period and site.
When grown for pulp or firewood, wood is mechanically harvested in winter when the leaves have shed and before the sap flow resumes. After chipping, wood chips must be stored to dry before burning. Green biomass (leaves) is unsuitable for combustion because it leads to corrosion of the boilers due to the high potassium content. Moreover, harvesting leaves also causes large nutrient losses from the site. In the future, wood from short rotation coppicing may also be used for the production of bioethanol or fine chemicals.
The environmental impact of short rotation coppice is greater than that of other, less intensive forestry systems, but lower than that of arable farming systems. Compared to high forest, there are greater losses of base cations, especially calcium, because a relatively large fraction of living bark tissue containing high nutrient concentrations is harvested. Short rotation coppice requires less fertilization, biocides and tractor movements than arable farming, and causes less soil disturbance. Short rotation coppice is therefore particularly applicable if there is a link with other functions, such as the capture of particulate matter along roads, or creating stepping stones for biodiversity. For the greenhouse gas balance and land use impact of short rotation coppice, see Lettens et al. (2003) and Garcia Quijano et al. (2005). For further information on energy crops, see Kuiper (2003) and Kuiper and Jansen (2002).
46.3 Coppice-with-standards
Coppice-with-standards is a management system in which coppice is combined with larger trees within the same stand (Photo 46-1; Figure 46-4). The upper tree layer consists mostly of trees of different ages and is referred to as the reserve.

Photo 46-1: A coppice-with-standards in Gerendal (NL). In the front, the coppice has just been cut (remaining coppice in the background). A number of ash trees have been spared and added as youngest generation to the reserve. The large oak tree shows the broad and low crown typical to the older trees in the reserve of coppice-with-standards. Photo J. den Ouden.
Coppice-with-standards has been a common management system in Europe, especially on richer soils, however, it has hardly been explicitly mentioned in historical sources (Tack et al., 1993; Verboven et al., 2006). In any case, the coppice-with-standards system was much more common than the limited extent in which it can still be found; it is still relatively common in France (Taillis sous futaie) and some parts of Germany (Mittelwald). Because the understorey is shaded by the reserve, the coppice layer usually consists of shade-tolerant species such as Carpinus, Corylus, Castanea and Alnus. For the reserve, species with good wood properties and transparent crowns are used such as Quercus, Populus, Fraxinus, or Larix. The trees in the reserve must also be sufficiently stable and able to tolerate free growth.
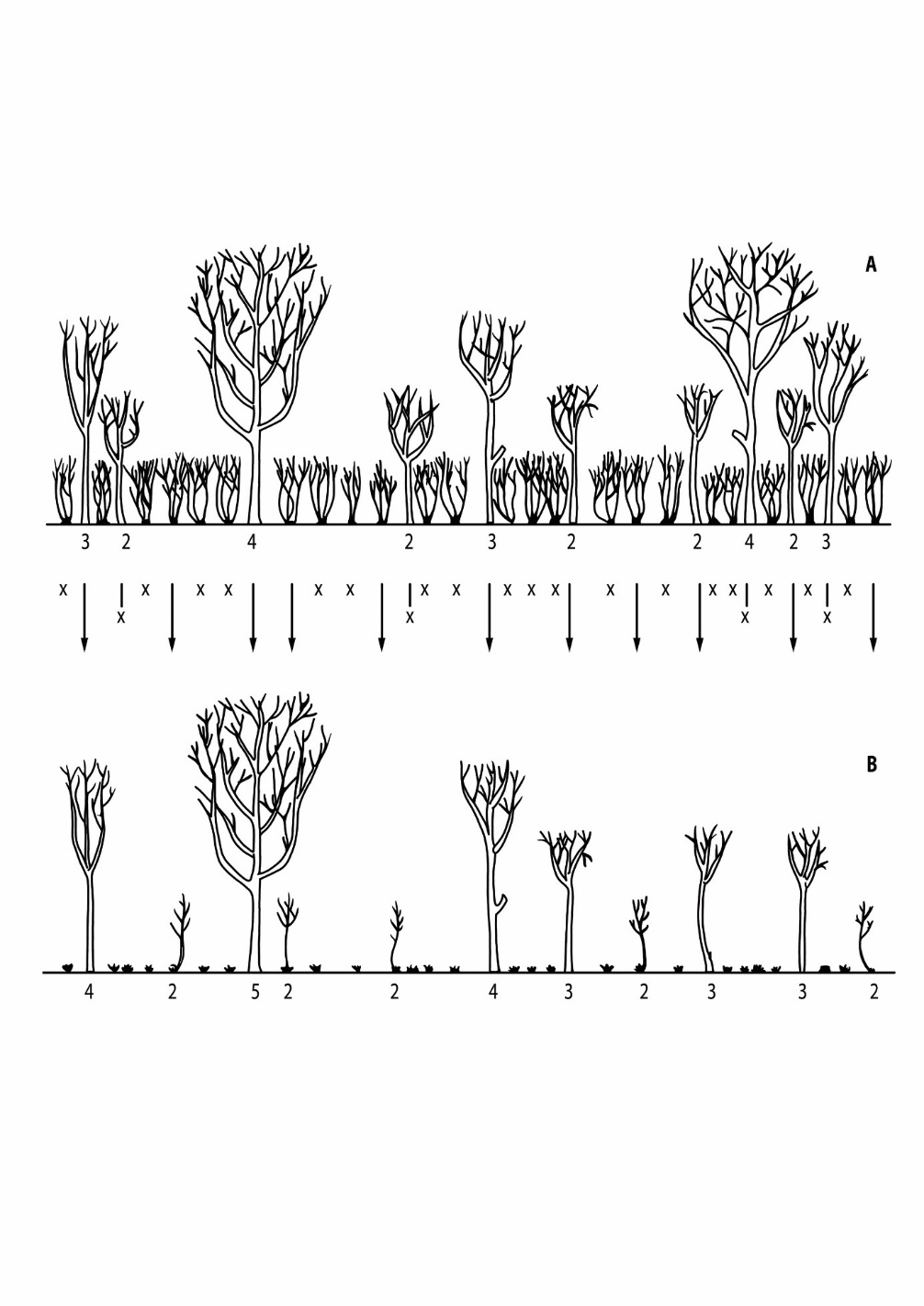
Figure 46-4: Schematic overview of a coppice-with-standards system with reserve trees (standards) in different age classes (indicated by numbers). In this example, trees are kept in the reserve for five generations, with the distance between generations equal to the cutting cycle of the coppice. In the upper part (A), four generations of trees can be seen just before felling (fifth generation not drawn), with a total of two fourth-generation standards, three third-generation standards, and five second-generation standards drawn. With a felling cycle of 20 years, these trees are 80, 60 and 40 years old respectively, just before the coppice is cut. The crosses indicate trees that are removed from the reserve, the arrows indicate trees that are kept. After felling (B), one standard grows from the fourth into the fifth generation, and five trees are added to the reserve and now represent the second generation.
The coppice layer is even-aged, while the reserve predominantly consists of trees of differing age classes that are a multiple of the rotation time within the coppice. With each felling, a number of trees from the coppice layer is spared and added to the reserve (Figure 46-4). These trees are best selected from naturally established seedlings, but can also be obtained from singling a stool, or planted separately between the coppice stools. Singled stems may not always provide suitable trees for the older reserve because of their reduced vigour in later life, the crooked trunk bases, and the increased risk of disease and death due to rotting of the stool. Therefore, the best options for the reserve are shoots growing from very young stools.
The felling of the reserve trees usually takes place simultaneously with the coppice harvest, or in the following year. A fixed ratio in stem numbers of the different age or circumference classes is aimed for in the reserve. In this way, the reserve’s structure is similar to the age or size distribution of a selection forest (section 39). Conceptually, coppice-with-standards management can therefore be regarded as the lowland alternative of the selection forest, but mainly with light demanding species. The total number of stems retained in the reserve depends on the relative importance of the products from the coppice versus the reserve and the shade tolerance of the coppice layer. Good growth and a high understorey density are important for the young reserve trees to develop straight stems and ensure branch free boles in the older reserve.
Table 46-2: Scheme for maintaining standards of the different age classes in the reserve of a coppice-with-standards system at two different growth levels. The graph shows the corresponding stem diameter distribution. In both situations, the intervention takes place with a basal area before felling of 17.4 m2 ha–1 and after felling of 7.2 m2 ha–1. This corresponds to a total reserve crown cover of 75% before felling and 31% after felling. Note that in the first age class, 10% mortality is taken into account and therefore slightly more trees are saved than strictly necessary. Data from De Turckheim & Brucchiamacchie (2005).
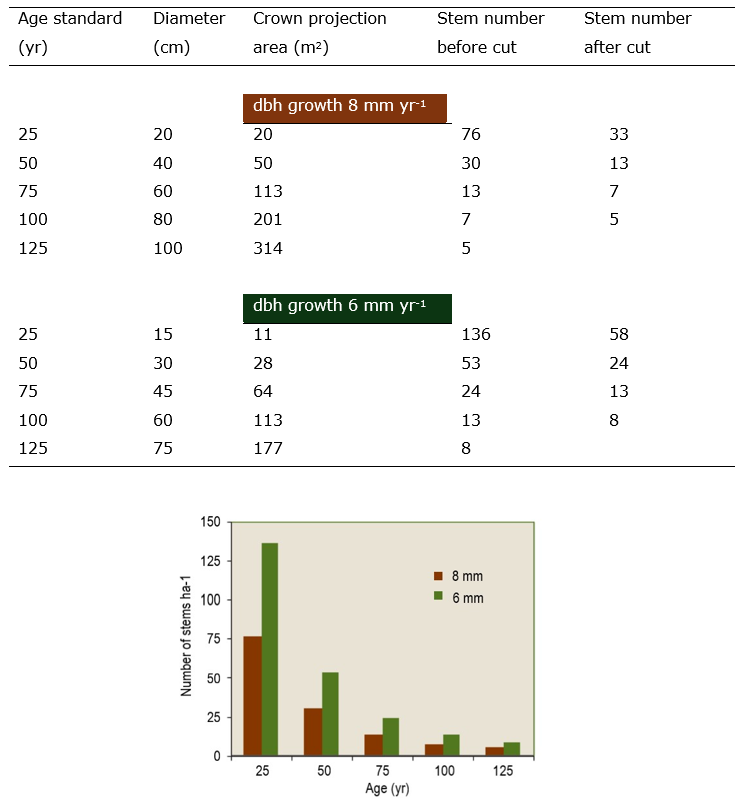
The literature notes a variety of stem numbers and ratios between age classes to be maintained in the reserve. According to Boppe and Jolyet (1901), trees of successive generations should be maintained in the ratios of 50 (youngest reserve), 30, 20 and 10 (oldest reserve). In France at Le Nouvion, a reserve ratio of 80, 40, 15, 5 oak trees is maintained over a hornbeam coppice layer in a 30-year cycle (Decocq et al., 2004). These ratios in the reserve are maintained by cutting a fixed share within each age class after each coppice cycle (Table 46-2). In the first example, 20, 10 and 10 trees respectively are harvested from the three younger classes, the oldest class is completely cut, and 50 new trees are added to the reserve. Diseased and dead trees, or trees with poor shape, are selected for felling so that growth remains concentrated in trees of the best quality. Care must be taken to ensure that the reserve is spatially distributed as evenly as possible over the plot. For an extensive treatment of coppice-with-standards, see Turckheim and Bruciamacchie (2005), Bärnthol (2003), and Volmuth (2021).
Economic changes have caused a gradual shift in the need for specific wood dimensions, leading to a subsequent shift in the coppice-with-standards system from an emphasis on coppicing to the maintenance of an increased proportion of larger trees (Bastien, 1998). Ultimately, due to the disappearance of the markets for coppice products and the rise in labour costs, the area of coppice-with-standards systems has greatly diminished and has often been transformed into high forest (Figure 46-5).
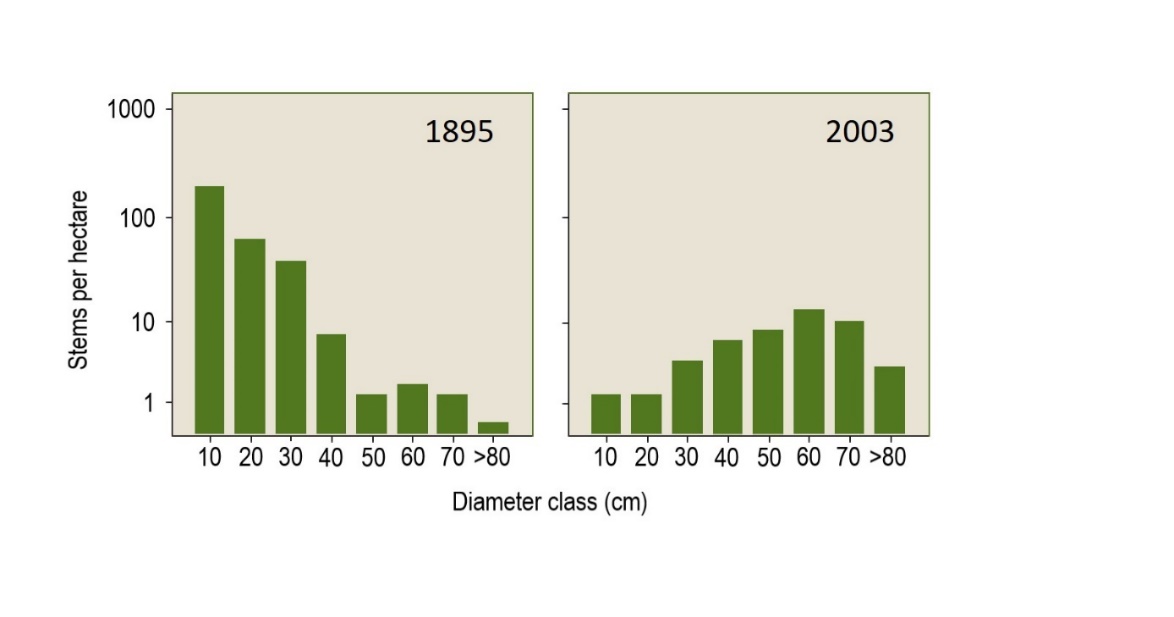
Figure 46-5: Changes in diameter distribution for pedunculate oak in a coppice-with-standards stand in Grotenhout (B) between 1895 and 2003. The 1895 distribution is typical of a coppice-with-standards stand with many young trees and a small number of larger trees in the reserve, and is very similar to the distribution of a selection forest. After the coppice-with-standards system was abandoned, the oaks continued to grow, leaving only relatively heavy oaks with hardly any regeneration of new oaks by 2003. After Nagels (2004).
46.4 Natural values in coppice and coppice-with-standards
Forests managed as coppice and coppice-with-standards may have high natural values. They often represent old forest habitats and have unique temporal light and nutrient dynamics that create favourable conditions for specific plant species. Moreover, old stools may form a substrate with associated species that are hardly found elsewhere, as is the case for ash coppice that harbours a number of rare moss species specific to the nutrient-rich, pH-neutral bark on the stools (Barkman, 1958).
Coppice forests have different dynamics to both natural and managed high forests (see Peterken, 1993; Buckley, 1992). Due to the short rotation, the understorey undergoes a rapid alternation between light and dark phases (Figure 46-6). In the older and closed phases of the coppice cycle, the soil is virtually bare due to the heavy shade. Following a harvest, full solar radiation reaches the soil, resulting in high light availability, strongly fluctuating temperatures, and increased evaporation. Repeated felling and soil disturbance and accelerated litter decomposition after clearing prevent the formation of a thick litter layer, which is beneficial for species regenerating from seed. The accelerated litter decomposition reduces the production of humic acids. The regular exposure of deeper soil layers (if old stools are dug up) prevents rapid soil acidification and stimulates the formation of mull humus. Directly following a coppice harvest, many species that depend on a high-light environment can establish. Individuals of shade-tolerant species can also increase in number and cover when the canopy reaches closure (Buckley, 1992; Peterken, 1993; Hermy & Vandekerkhove, 2004; Rackham, 2006).
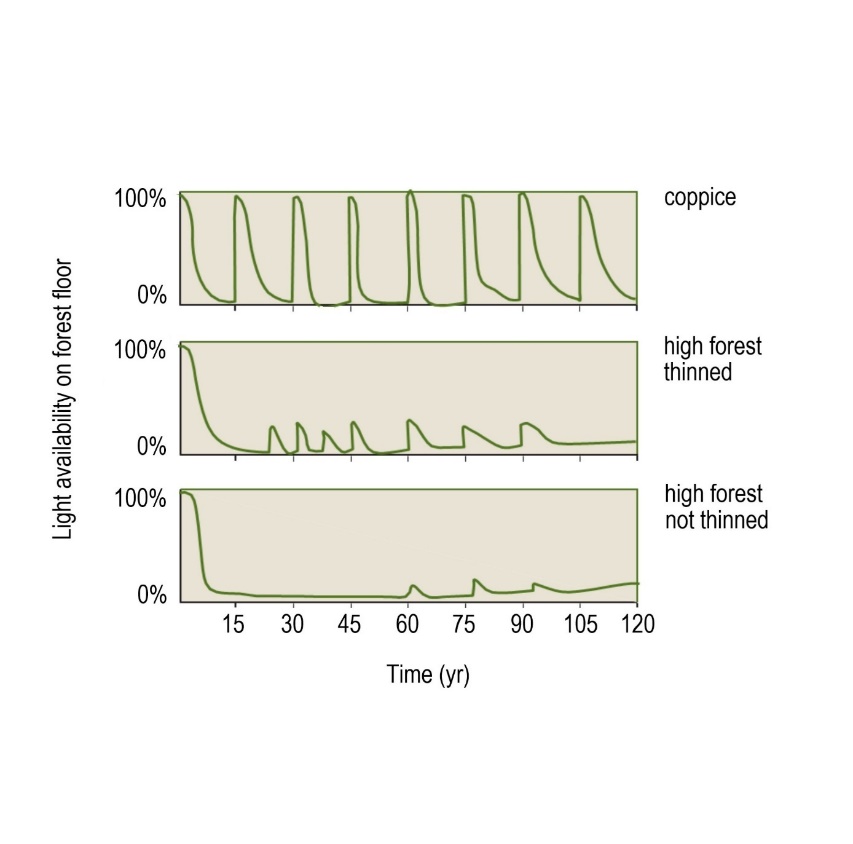
Figure 46-6: Schematic representation of the change in light availability over time in oak forest managed as coppice in a 15-year rotation, a thinned high forest, and an unthinned high forest. In the thinned forest, thinning occurs in the early phases of stand development and then decreases in frequency. In the unthinned forest, the temporary increases in light availability are caused by tree mortality and/or disturbances.
The typical alternation of light and dark periods in coppice culture and the subsequent change in vegetation was described by Salisbury (1924, p. 21) as “(…) a continual rise and fall in the vigour of the shade flora accompanied by an ebb and flow of the marginal flora.” This explains the occurrence of a diversity of regenerative strategies in coppice systems (Figure 46-7). Light-dependent and wind-dispersed species such as fireweed (Chamerion angustifolium) only occur in the first years after felling. Perennial species with a long-lived seed bank germinate after felling, but disappear again when the coppice canopy fully closes. On acidic soils, this concerns both ancient-forest species such as slender St. John’s-wort (Hypericum pulchrum) and species from more open areas such as broom (Cytisus scoparius) and heather (Calluna vulgaris). Finally, in coppice forests we find perennial, (moderately) shade-tolerant species that can persist in small patches and expand vegetatively when the conditions are (again) favourable. Examples are bracken fern (Pteridium aquilinum), bilberry or blueberry (Vaccinium myrtillus) and Solomon’s seal (Polygonatum multiflorum). True forest plants such as wood anemone (Anemone nemorosa) and yellow archangel (Galeobdolon luteum) also benefit from the regular higher light availability in coppice due to increased growth and seed production.
Figure 46-7: Occurrence of four species groups of higher plants in a coppice felling cycle in England. The coppice mainly consisted of hazel, with a mixture of various other species. After Ash & Barkham (1976).
A similar succession of species also applies to birds and butterflies (Buckley, 1992; Hermy & Vandekerkhove, 2004). Rackham (2006) and Decocq et al. (2005) list species for England and Le Nouvion (France) respectively that have a strong affinity with coppice. These studies involve coppice and coppice-with-standards systems on predominantly nutrient-rich soils and therefore carry a more species-rich vegetation, often with a striking number of vernal species such as common primrose (Primula vulgaris) and bluebell (Scylla non-scripta). In association with the plant species and structures occurring in the coppice cycle, other taxa may also profit from coppiced forests, like the scarce fritillary butterfly (Euphydryas maturna), hazel grouse (Tetrastes bonasia) and hazel dormouse (Muscardinus avellanarius) (Unrau et al., 2018)
It is difficult to determine which species can be regarded as typically associated with coppice and coppice-with-standards, because most of these systems have been converted or developed into high forest. Moreover, the relation between species and coppice also applies to their dependence on historic forest sites. Vice versa, many old forest species are again dependent on the disturbance regime associated with (traditional) forest management, so that their rarity is not only caused by the small and fragmented area of historically old forest, but also by changes in management and environmental quality (Wulf, 1997; Honnay et al., 1999; Van Calster et al., 2008a; Volmuth, 2021).
Due to the continuous deep shade and accumulation of litter and the associated acidification, in old high forests many of the typical old forest species disappear (Bijlsma, 2002; Volmuth, 2021). Moreover, the lack of regular disturbance leads to the disappearance of species from the seed bank (Van Calster et al., 2008b). When management by coppicing, or coppice-with-standards is resumed, many species will have to come from elsewhere, but are hindered by a limited dispersal capacity. The habitat may also have become unsuitable due to the increasingly acidified soil (Baeten et al., 2009), on top of the previous reduction in soil fertility due to the loss of nutrients after continuous extraction of stems, bark and leaves (Šrámek et al., 2015). However, nutrient-depletion effects due to coppicing are not unequivocal (Hölscher et al., 2001). Furthermore, a small number of highly competitive species can dominate the undergrowth as a result of the sudden release of nutrients from the accumulated litter layer, making the establishment of the desired species impossible.
Further reading
Boer (1857); Buckley (1992); Danfors et al. (1998); De Turckheim & Bruchiamacchie (2005); Harmer & Howe (2003); Jansen & Kuiper (2001); Peterken (1993); Rackham (2006); Tack et al. (1993); Unrau et al. (2018); Volmuth (2021).
References
Ash, J.E. & J.P. Barkham 1976. Changes and variability in the field layer of a coppiced woodland in Norfolk, England. Journal of Ecology, 64: 697-712.
Baeten, L., B. Bauwens, A. De Schrijver, L. De Keersmaeker, H. Van Calster, K. Vandekerkhove, B. Roelandt, H. Beeckman, & K. Verheyen 2009. Herb layer changes (1954-2000) related to the conversion of coppice-with-standards forest and soil acidification. Applied Vegetation Science, 12: 187-197.
Barkman, J.J. 1958. Phytosociology and ecology of cryptogamic epiphytes, including a taxonomic survey and description of their vegetation units in Europe. Assen: Van Gorcum.
Bärnthol, R. 2003. Nieder- und Mittelwald in Franken. Waldwirtschaftsformen aus dem Mittelalter. Verlag Fränkisches Freilandmuseum, Bad Windsheim.
Bastien, Y. 1998. Eléments de gestion forestière. Nancy: ENGREF.
Bijlsma, R.J. 2002. Bosrelicten op de Veluwe. Een historisch-ecologische beschrijving. Rapport 647. Wageningen: Alterra.
Boer, R.W. 1857. Bijdragen tot de kennis der houtteelt. Zwolle: Tjeenk Willink.
Boppe, L. & A. Jolyet 1901. Les forêts. Traité de silviculture. Paris: J.-B. Baillière et fils.
Buckley, G.P. (ed.) 1992. Ecology and management of coppice woodlands. London: Chapman & Hall.
Buis, J. 1985. Historia Forestis: Nederlandse bosgeschiedenis. Utrecht: HES Uitgevers.
Crockford, K.J. & P.S. Savill 1991. Preliminary yield tables for oak coppice. Forestry, 64: 30-48.
Danfors, B., S. Ledin & H. Rosenqvist 1998. Short-rotation willow coppice. Growers’ manual. Uppsala: Swedish Institute of Agricultural Engineering.
Decocq, G., M. Aubert, F. Dupont, D. Alard, R. Saguez, A. Wattez- Franger, B. De Foucault, A. Delelis-Dusollier, & J. Bardat 2004. Plant diversity in a managed temperate deciduous forest: Understorey response to two silvicultural systems. Journal of Applied Ecology, 41: 1065-1079.
Decocq, G., M. Aubert, F. Dupont, J. Bardat, A. Wattez-Franger, R. Saguez, B. De Foucoult, D. Allard & A. Delelis-Dusollier 2005. Silviculture-driven vegetation change in a European temperate deciduous forest. Annals of Forest Science, 62: 313-323.
Den Ouden, J. & Th. Spek (red.) 2007. Ontstaanswijze van eikenclusters in het natuurterrein De Wilde Kamp bij Garderen: landschapsgeschiedenis, bodemontwikkeling en vegetatiegeschiedenis. Rapportage Archeologische Monumentenzorg 131B. Amersfoort: RACM.
De Türckheim, B. & M. Bruciamacchie 2005. La futaie irrégulière: Théorie et pratique de la sylviculture irrégulière, continue et proche de la nature. Aix-en-Provence: Edisud.
Gannevat, M.F. 1950. Courbes et tables de croissance. Revue Forestière Française, 2: 223-226.
Garcia-Quijano, J.F., G. Deckmyn, E. Moons, S. Proost, R. Ceulemans & B. Muys 2005. An integrated decision support framework for the prediction and evaluation of efficiency, environmental impact and total social cost of domestic and international forestry projects for greenhouse gas mitigation: Description and case studies. Forest Ecology and Management, 207: 245-262.
Harmer, R. 2001. Growth of coppice shoots following felling of maiden oaks at different heights above ground. Quarterly Journal of Forestry, 95: 217-233.
Harmer, R. & J. Howe 2003. The silviculture and management of coppice woodlands. Edinburgh: Forestry Commission.
Hermy, M. & K. Vandekerkhove 2004. Bosgebieden. In: M. Hermy, G. De Blust & M. Slootmaekers (eds.), Natuurbeheer (pp. 307-359). Leuven: Davidsfonds
Hölscher, D., E. Schade & C. Leuschner (2001). Effects of coppicing in temperate deciduous forests on ecosystem nutrient pools and soil fertility. Basic and Applied Ecology, 2: 155–164.
Honnay, O., M. Hermy & P. Coppin (1999). Impact of habitat quality on ancient forest plant species recolonisation. Forest Ecology and Management, 115: 157-170.
Hundeshagen, J.C. 1826. Die Forstabschätzung auf neuen, wissenschaftlichen Grundlagen; nebst einer Charakteristik und Vergleichung aller bisher bestandenen Forsttaxations-Methoden. Laupp, Tübingen.
Jansen, P. & L. Kuiper 2001. Hakhout. Suggesties voor het beheer. Wageningen: Stichting Bos en Hout.
Kuiper, L. 2003. Samenvatting van de resultaten van zes jaar onderzoek naar energieteelt. Wageningen: Centrum voor Biomassa Innovatie.
Kuiper, L. & P. Jansen 2002. Bos en energie. Zeist: Bos en Hout/ Stichting Probos.
Lettens, S., B. Muys, R. Ceulemans, E. Moons, J. Garcia & P. Coppin 2003. Energy budget and greenhouse gas balance evaluation of sustainable coppice for electricity production. Biomass and Bioenergy, 24: 179-197.
Mitchell, C.P., J.B. Ford-Robertson, T. Hinckley & L. Sennerby-Forsse, L. (eds.) 1992. Ecophysiology of short rotation forest crops. London: Elsevier Applied Science
Nagels, A. 2004. Vergelijking van de historische en actuele bestandsopbouw in het Grotenhout bij Turnhout. Leuven: Faculteit Landbouwkundige en Toegepaste Biologische Wetenschappen, K.U.
Peterken, G.F. 1993. Woodland conservation and management (2de ed.). London: Chapman & Hall.
Rackham, O. 2003. Ancient woodland, its history, vegetation and uses in England. Kirkcudbrightshire: Castlepoint Press.
Rackham, O. 2006. Woodlands. London: Collins.
Rossier, G. & W. Micuta 1999. Should charcoal braziers be promoted? In: J. Poker & I. Stein (eds.), Forests in focus. Proceedings Forum Forests and Energy (pp. 62-64). Schneverdingen: Alfred Toepfer Akademie für Naturschutz.
Salisbury, E.J. 1924. The effects of coppicing as illustrated by the woods of Hertfordshire. Trans. Hertfordshire Natural History Society, 18: 1-24.
Schaars, A.H.G. 1974. De bosbouw van het ‘Entel’ in de tweede helft van de achttiende eeuw. Zutphen: De Walburg Pers.
Šrámek, M., D. Volařík, A. Ertas & R. Matula (2015). The effect of coppice management on the structure, tree growth and soil nutrients in temperate Turkey. Journal of Forest Science, 61: 27-34.
Tack, G., P. Van Den Bremt & M. Hermy 1993. Bossen van Vlaanderen. Een historische ecologie. Leuven: Davidsfonds.
Unrau, A., G. Becker, R. Spinelli, D. Lazdina, N. Magagnotti, V.-N. Nicolescu, P. Buckley, D. Bartlett & P. D. Kofman 2018. Coppice Forests in Europe. Albert Ludwig University of Freiburg, Freiburg im Breisgau, Germany.
Van Calster, H., L. Baeten, K. Verheyen, L. De Keersmaeker, S. Dekeyser, J.E. Rogister & M. Hermy 2008a. Diverging effects of overstorey conversion scenarios on the understorey vegetation in a former coppice-with-standards forest. Forest Ecology and Management, 256: 519-528.
Van Calster, H., R. Chevalier, B. Van Wyngene, F. Archaux, K. Verheyen, & M. Hermy 2008b. Long-term seed bank dynamics in a temperate forest under conversion from coppice-with-standards to high forest management. Applied Vegetation Science, 11: 251-260.
Verboven, H., K. Verheyen & M. Hermy 2006. Oude bossen als natuur- en cultuurhistorisch erfgoed: landschapsherstel versus natuurherstel? Een gevalstudie: Grotenhout in Turnhout. Monumenten & Landschappen, 25: 6-32.
Vollmuth, D. 2021. Die Nachhaltigkeit und der Mittelwald. Göttinger Forstwissenschaften Band 10. Göttingen:. Universitätsverlag Göttingen.
Wulf, M. 1997. Plant species as indicators of ancient wood-land in northwestern Germany. Journal of Vegetation Science, 8: 635-642.
Glossary:
|
assortment |
A certain dimension of a log or part thereof, usually determined by the specific application of the wood |
|
coppice |
Silvicultural system in which woody plants are regenerated vegetatively by regrowth of shoots from stumps. |
|
coppice-with-standards |
A coppice system in which at each felling a number of stems are retained as standards to form an uneven-aged overstorey |
|
coupe |
A section within a coppice woodland cut entirely after a given period (rotation) |
|
cutting |
A short piece of shoot taken from a plant and planted in soil where it will produce adventitious roots, eventually creating vegetative offspring from the original plant. |
|
faggot |
A bundle of branches or twigs, to be used as firewood. |
|
fascine mattress |
Bundles of long twisted willow shoots tied into mats for use in water works. |
|
planting stock |
The seedlings used for planting; they can be grown from seed or propagated vegetatively |
|
pollard |
A coppice stool placed at some height above the ground to prevent browsing by animals or flooding. |
|
reserve |
The population of high trees (standards) in a coppice-with-standards |
|
shredding |
The repeated removal of branches from a tree, leaving only a part of the upper crown |
|
spacing interval |
The distance between trees in a stand |
|
stère |
A 1 m3 stack of wood |
|
stool |
The structure emerging when shoots are repeatedly cut. |
|
tanbark |
Bark, usually from oak, used for tanning leather |
Acknowledgements
This Chapter is published on the EUROSILVICS platform, established as part of the EUROSILVICS Erasmus+ grant agreement No. 2022-1-NL01-KA220-HED-000086765.
This text was originally prepared for the Dutch textbook “Bosecologie en Bosbeheer”.
Author affiliation:
|
Jan den Ouden |
Forest Ecology and Forest Management Group, Wageningen University and Research, Droevendaalsesteeg X, P.O. Box XX, XXXX YY Wageningen |
|
Patrick Jansen |
Stichting Probos, Hollandseweg 7j, 6706KN Wageningen |
|
Linda Meiresonne |
Research Institute Nature and Forest, Herman Teirlinckgebouw, Havenlaan 88, 1000 Brussel |